Solutions
Provide strategic advantage for clinical trial supply solutions
Global sourcing services
Purchasing control drugs/reference preparations for customers is the core business of Clinvantage.

Clinical trial supply chain management
Clinvantage has extensive experience in clinical trial supply chain management.

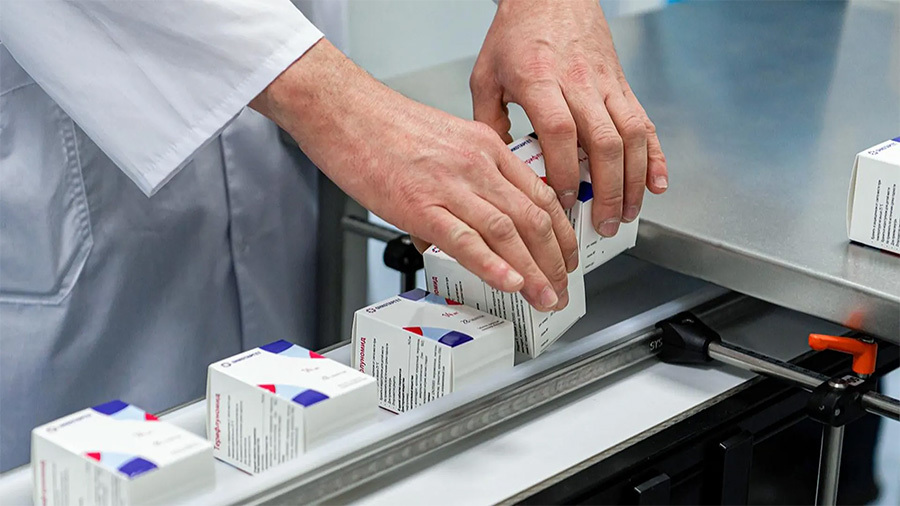
Packaging solutions for Clinical Trials Drug
The packaging design of drugs for clinical trials is very important for drug management and prevention of blindness.

Global supply services for clinical trials
Our partner warehouse supports our customers' clinical trial projects in key regions around the world.

Clinvantage Pharmaceutical Technology Co.,Ltd.
Clinical Trial Supply Management Center
Clinvantage Pharmaceutical Technology Co., Ltd. (referred to as "Clinvantage") was established in 2016, with its headquarters located in the Life Science Park in Changping District, Beijing. The company focuses on providing clinical trial supply management services for global pharmaceutical research and development enterprises, with a registered capital of 40 million RMB.
Clinvantage adheres to the core philosophy of "accompanying and helping clients achieve their goals," and is committed to offering integrated, customer-satisfactory clinical trial supply management services. Through our efforts, we aim to help clients build strategic competitive advantages and accelerate the success of clinical trial projects and drug development.

Services
Have a professional and perfect service unit matrix
Insights
Professional to promote the growth of the industry
The "Specifications for the Supply and Management of Drugs Used in Clinical Trials of Biological Products," jointly issued by the China Vaccine Industry Association and the China Standardization Association, was developed and drafted with the participation of Huaren Pharmaceutical Clinvantage. It is a normative standard document in China for the supply and management of drugs used in clinical trials of biological products.
The "Specifications for the Supply and Management of Drugs Used in Clinical Trials of Biological Products," jointly issued by the China Vaccine Industry Association and the China Standardization Association, was developed and drafted with the participation of Huaren Pharmaceutical Clinvantage. It is a normative standard document in China for the supply and management of drugs used in clinical trials of biological products.
The "Specifications for the Supply and Management of Drugs Used in Clinical Trials of Biological Products," jointly issued by the China Vaccine Industry Association and the China Standardization Association, was developed and drafted with the participation of Huaren Pharmaceutical Clinvantage. It is a normative standard document in China for the supply and management of drugs used in clinical trials of biological products.
The "Specifications for the Supply and Management of Drugs Used in Clinical Trials of Biological Products," jointly issued by the China Vaccine Industry Association and the China Standardization Association, was developed and drafted with the participation of Huaren Pharmaceutical Clinvantage. It is a normative standard document in China for the supply and management of drugs used in clinical trials of biological products.
Contact Us
Clinvantage has an integrated solution for clinical trial supply.
Corporate E-mail

Business Address
Building 3, Yuanda Science Park, Liandong U Valley, 18 Shuanghe Street, Shunyi District, Beijing
In order to facilitate better communication between Clinvantage and you, you can leave your phone number or E-mail address.